Our Science
Alzheimer dementia
Our flagship R&D program aims to develop an all-new HFN cell therapy for Alzheimer dementia based on synaptic regeneration.
For a detailed scientific background, see Chief Scientific Officer Prof Valenzuela’s highly-cited review article here.
S2N side-steps the amyloid hypothesis that has produced controversial outcomes over the years. Our approach is entirely different. Our target is the ultimate cause of dementia: lost neurons and synapses. We do this by microinjecting a patient’s own HFN cells directly into the hippocampus, the memory centre of the brain and first area to be devastated by Alzheimer’s.
We have completed two rodent studies showing that skin-derived neuroprecursors can reverse age-related memory deficits, rescue depleted synapses and restore network function. Donor cells survive well in the rodent brain, differentiating into anatomically correct neurons that integrate into host memory circuits.
Over the past 8-years, we have also pioneered a world-first Phase I/II veterinary trial of skin-derived neural precursor cell therapy for older pet dogs with Canine Dementia. Prof Valenzuela’s team helped define the syndrome and is a leader in this field.
For the first time, dogs with dementia were successfully treated, with the majority returning to normal. At the same time, synapses in the hippocampus increased several-fold, a stunning outcome despite the confirmed presence of amyloid and tau pathology. This work has now been published.
S2N is also interested in partnering with leading research groups to trial HFN cell therapy in Parkinson’s disease models and other models of neurodegenerative disease.
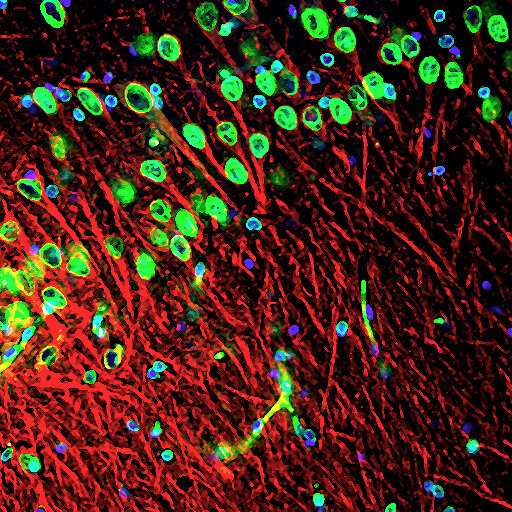
About the image: Skin from a dog was processed in our lab to produce neuronal precursor cells and then transplanted into the hippocampus of an old rat. Image show a microscope scan of the rat’s brain tissue 10 weeks later. There are many donor skin-derived neurons in green (nuclei), shooting off anatomically correct dendrites (red) to connect with the host brain.
Made to order neurons
A major advantage of HFN technology is that they only produce neurons. That’s right: no glia, no oligos… nothing else except wonderfully pure neurons.
Neuronal cell cultures can be used for all sorts of purposes, including drug discovery and toxicity testing, disease modelling and basic science.
Wouldn’t it be great if you could order your own customized neuronal line? That is one of the aims of S2N cell technology and our first commercial product under development is gabergic HFN-derived neurons.
Gabergic neuronal dysfunction is considered central to many psychiatric disorders, especially schizophrenia, and so we look forward to working together with researchers in this field.
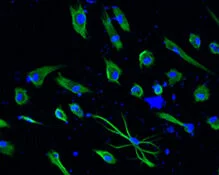
About the image: Human hair follicle-derived GAD67+ (green) neurons following S2N gabergic differentiation protocol.
Canine Dementia: the missing link
The canine brain (image upper) is a beautiful thing. The dog’s thinking neocortex is remarkably similar to the human brain (image lower), layered with hills and valleys (gyri and sulci) that rodents just don’t have (the rodent brain is smooth).
The canine brain’s hippocampus (memory centre) is also remarkable - it has a dorsal (upper) part very much like the rodent hippocampus (denoted by the crosshairs), and a ventral (lower) part very much like the human hippocampus.
So the canine brain really is a bridge between rodents and humans, and that is what has been missing in Alzheimer and dementia research for so long.
Scientists have have “cured” Alzheimer’s disease in mice more than 50-times over but none of those treatments have succeeded in human trials.
About 15% of older companion dogs also develop a dementia syndrome very similar to human dementia. They become forgetful, irritable, lost, stuck, wandering around aimlessly, often over and over. Their sleep patterns become disrupted and in later stages they fail to recognise their carers and soil in the house.
Canine dementia is devastating to the human-animal bond and almost always leads to euthanasia when quality of life falls too low and care burden too high.
Prof Valenzuela’s group helped define the clinical syndrome of Canine Dementia as well as understand its epidemiology and biology. Recently, his group has shown that Canine Dementia not only features classic Alzheimer amyloid plaques but also early-stage tau pathology, distributed in similar pattern to early stage human Alzheimer’s disease.
Given all these parallels, S2N is excited to be near completion of its DOGS+CELLS Trial, a world-first veterinary trial of skin-derived cell therapy.
Prof Valenzuela revealed positive topline results of the DOGS+CELLS Trial at Vet Expo in Sydney on October 28, 2020. Contact us for a copy of the slidedeck.
Hair follicle derived neural precursors.
Manufacture.
Regenerative medicine and cell therapies in particular have historically been slave to the mantra “the process is the product”.
S2N has broken those constraints by comprehensively defining HFN’s Critical Quality Attributes following FDA guidelines for purity, identity, safety, stability and potency.
Our 100% cGMP-compatible process delivers unrivaled cell quality and reliability: >90% purity across a multiplex panel of markers AND <10% line-to-line variability.
S2N is ready to support cell production for human clinical trials and commercial applications.
Biological Control.
The first step in the cell therapy journey is that donor cells need to get from their site of delivery to final destination in the brain. This process is called chemotaxis.
To date, there has been no practical method for in vivo migratory control of transplanted cells.
However, one of the stand-out features of HFNs is their chemotaxic responsiveness to a specific brain signal.
In the figure above, we measured HFN migration towards this signal and showed this can be abolished by a specific receptor blocker, proving that HFN migratory behaviour is mediated by this receptor.
We have used this insight to test if skin-derived neuroprecursors can be guided in the brain. How? By using a powerful endogenous upregulator of this signal.
In rodents, just a few weeks of signal upregulation around the time of engraftment was sufficient to increase donor cell survival and neuronal differentiation in the hippocampus by >50% and synaptic regeneration by >100%.
S2N is diving deep into this fascinating science and we are very excited about the potential for controlling donor cell destiny with a simple strategy that could be readily applied in the clinic.
Preclinical Trials.
Canine veterinary trial of our cell technology has been a strategic decision designed to move beyond rodent studies.
With this comes a need to more precisely measure and understand canine in-home behaviour. That is why Prof Valenzuela’s team developed Function Cloud, an all-new wearable device system that can track human or pet movement around the home.
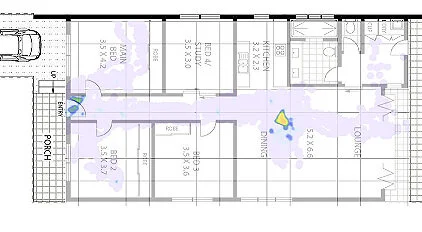
In the figure above, a 24-hour movement map of one of veterinary trial participants is overlaid on their floor plan. The grey zone was found to be remarkably consistent day-on-day, in effect a ‘spatial signature’ for that dog at baseline to be able to compare with at later stages.
The hot spot in the middle of the dining area shows where this dog routinely circles, over and over, a tell-tale sign of Canine Dementia.
S2N has exclusive access to Function Cloud technology for its large-animal veterinary trials and future human trials. We are currently applying machine learning algorithms to automatically extract clinically-relevant features from this big data.
S2N is also interested in partnering with leading research groups to trial HFN cell therapy in Parkinson’s disease models and other models of neurodegenerative disease.